CC1. What Is the Greenhouse Effect?

Chapter 1
Life on Earth would be impossible without the atmosphere. It contains oxygen and other gases essential for plants and animals. The atmosphere protects us from the Sun’s harmful rays and acts like a blanket to keep our planet at a livable temperature.
There is, however, some disturbing evidence that the heat-trapping property of our atmosphere is changing.
Life on Earth is possible because our atmosphere keeps our planet warm. This warmth is due to the “greenhouse effect,” which is a natural phenomenon.
Photo of New Guinea lowlands by Reginald Barrett.

Changing Climate is about scientific research that is going on today. Research on our planet’s climate is of such importance that whenever a new discovery is made, or a new theory is proposed, a story about it is carried in major newspapers. Among the most frequent topics appearing in the press during the past decade are global warming and the greenhouse effect.
Question 1.1. What is the difference between “climate change” and “global warming?”

Global warming refers to the fact that over the past century the average temperature of Earth has been gradually increasing. Nobody knows for certain if this trend will continue, and if it does, how much the temperature will rise. This is cause for concern because a further increase in Earth’s temperature may disrupt global systems worldwide, with effects ranging from more intense storms and floods in some regions to droughts and heat waves in others.
Major changes are not expected to occur tomorrow, or the day after tomorrow, or possibly even within our lifetime. The issue is rather how our actions today will affect the world of our children and grandchildren.
Despite the importance of global warming, many people are not aware of it, or have misconceptions. For example, some people believe that global warming is caused by the “hole” in the ozone layer, while others think it’s caused by black soot billowing from smokestacks. Neither is true. It’s a little more complicated, and has to do with something called the “greenhouse effect.”
The greenhouse effect is a natural phenomenon that makes our blue-green planet hospitable for life. The effect is caused by certain invisible gases—called greenhouse gases—in the atmosphere. Without those gases, which keep Earth warm, our planet would be a frigid ball of ice.
The reason many scientists believe the average temperature of Earth will continue to warm is because the concentration of greenhouse gases is increasing as a result of human activities. So, to understand the reasons why the globe may be warming up, and to predict what may happen in the future, we first have to understand the greenhouse effect.
I. Greenhouse Effect in a Greenhouse
Most of Earth’s atmosphere consists of nitrogen and oxygen. These gases allow sunlight to pass through them. They do not absorb heat from the Sun. However, the atmosphere also contains other gases that absorb heat. Sunlight warms them. Among these gases are carbon dioxide, water vapor, and methane, all of which existed in the atmosphere long before there were human beings, and are responsible for giving Earth a warm, comfortable climate. How do they do this? A simple greenhouse provides a clear analogy.
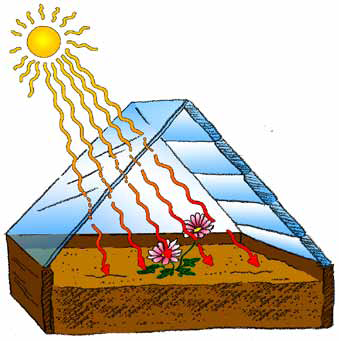
A. Sunlight consists of visible light and heat rays (called infrared energy). When sunlight falls on the glass window of a greenhouse, the visible light passes through, but some of the heat rays are absorbed, warming the glass. The rest of the heat rays pass through the glass and warms the soil and plants inside.
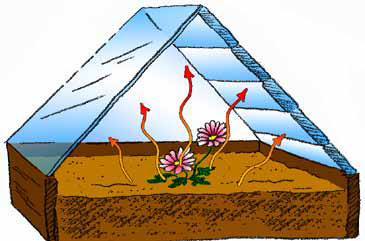
B. As the soil and plants warm, they give off heat (infrared energy). This heat energy is absorbed by the glass windows of the greenhouse, warming the interior even more. (You can feel heat energy if you put your hand next to any object that has been in the Sun all day.)
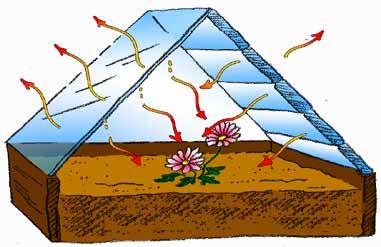
C. As the windows of the greenhouse warm, they also give off heat energy. Some of that energy escapes to the air outside, but some of it goes back into the greenhouse to warm the plants and soil even more.
The interior of the greenhouse gets warmer and warmer, until finally the amount of heat that escapes to the outside air equals the amount of heat flowing into the greenhouse from the Sun and the warm glass window.
You may be familiar with this effect when you enter a car that has been in the sunlight for a few hours with the windows closed. The glass windshield traps heat, so the air inside the car is warmer than the air outside the car.
II. Earth’s Greenhouse Effect
There are two ways in which this effect in the greenhouse and car is different from what occurs in Earth’s atmosphere. First, imagine you open the door to the greenhouse (or car). Warm air rushes out and is replaced by cooler air. This exchange of air is what cools the greenhouse. Can you imagine cooling Earth this way?
Second, in a greenhouse, the special heat-trapping material—the glass—is a solid surrounding the greenhouse. In the atmosphere, carbon dioxide, water vapor, and methane are spread thinly throughout the atmosphere.
In other words, Earth heats up like a greenhouse, but it is not actually a greenhouse. Because the carbon dioxide, water vapor, and methane act like the glass in a greenhouse, they are called greenhouse gases.
The diagrams on this page illustrate the greenhouse effect in Earth’s atmosphere. Compare it with the illustrations on the previous page to see how Earth’s atmosphere system differs from an actual greenhouse.
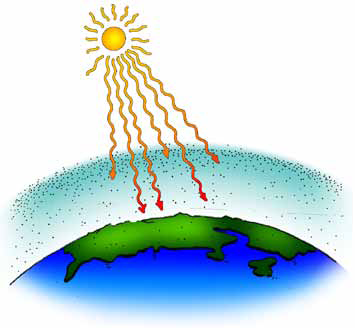
A. Energy from the Sun warms the atmosphere and the surface of Earth.
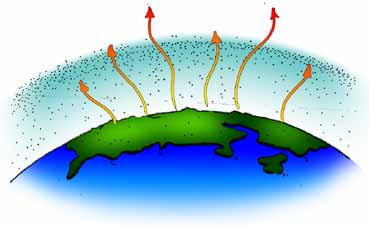
B. The warm soil, rocks, and water on Earth’s surface give off heat energy, warming carbon dioxide and other greenhouse gases in Earth’s atmosphere.
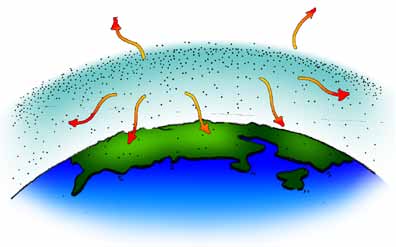
C. Some of the heat energy escapes from the atmosphere into space, but some of it returns to Earth to warm it further.
In a sense, the greenhouse gases act like a blanket, trapping heat energy near Earth’s surface. These gases keep Earth’s surface about 33°C (60°F) warmer than it would otherwise be.
If there were no greenhouse gases in our atmosphere, our entire planet would be so much colder than it is today—the oceans and all water would be completely frozen.
Liquid water was very important in the origin of life on Earth. If there were no greenhouse effect, it is likely that life—including us, of course—would not exist.
Question 1.2. What is the difference between “global warming” and “the greenhouse effect?”
III. Who Discovered the Greenhouse Effect?
More than 100 years ago, Jean Fourier realized the atmosphere possessed heat-trapping properties. He coined the term greenhouse effect to refer to the idea that the atmosphere acted somewhat like the glass walls of a greenhouse, allowing sunlight to enter but preventing some of the heat energy escaping into space. This effect is due to the water vapor, methane, and carbon dioxide that exist naturally in the atmosphere.
For billions of years varying levels of carbon dioxide have been maintained in the atmosphere.
Carbon dioxide was added to the atmosphere naturally by volcanic action, animal respiration, and the decomposition and burning of forests. Carbon dioxide was also removed naturally from the atmosphere by absorption in the oceans, or it was incorporated into trees and other plant life. In this way a balance was maintained.

In 1896, the Swedish chemist Svante Arrhenius, who was familiar with Fourier’s ideas, published an article with dire predictions. Arrhenius knew the capacity of the atmosphere to trap heat was due to the greenhouse gases that existed naturally in the atmosphere. He was also aware the concentration of one of those gases, carbon dioxide (the same invisible gas that makes bubbles in soft drinks), was increasing. He decided this increase in carbon dioxide in the atmosphere was a result of the way merchandise was being produced—the industrial revolution was under way—irreversibly affecting society and the environment.
The industrial revolution is the name given to the changes in the production of goods and means of transportation that began when the steam engine substituted steam power for muscle power. In 1769 the first practical steam engine was patented by the Scottish instrument maker James Watt. Although no one knew it at the time, this invention started the industrial revolution.
Prior to the industrial revolution animals and men were the basic sources of energy. Inventions like the steam engine made large factories possible and changed the landscape of our country from farms and small towns to huge cities with industrial areas connected by rails and highways. By the time of Arrhenius, vast quantities of coal were routinely burned to provide energy to run factories. As a result, air pollution was becoming a problem throughout Europe.
Arrhenius realized that when coal burned it released not only thick black smoke; it also released the invisible gas, carbon dioxide. He also realized that vast quantities of carbon stored in the coal for millions of years was now being released into the atmosphere as CO2.
Based on his knowledge of the properties of carbon dioxide, and Fourier’s ideas about the greenhouse effect, Arrhenius predicted the concentration of carbon dioxide in Earth’s atmosphere would eventually double, and when it did, the result would be an increase in average global temperatures of up to 5°C (9°F).
While the addition of just a few degrees may not seem like much compared with the natural greenhouse effect of 33°C (60°F), the effect of such a change on human society can make a huge difference.

IV. Can You Explain the Greenhouse Effect?
A 2012 study done by University of California Education Dept. and Psychology Dept. members, Michael Ranney, Dav Clark, Daniel Reinholz, and Sarah Cohen, shows that reading a brief, 400-word text yields both large knowledge gains about the mechanism of global warming and more climate change acceptance. The study involved asking people to answer the question, “How would you explain global warming’s mechanism?”
Here is the 400-word answer:
“How does climate change (“global warming”) work?”
Scientists tell us that human activities are changing Earth’s atmosphere and increasing Earth’s average temperature. What causes these climate changes?
First, let’s understand Earth’s “normal” temperature: When Earth absorbs sunlight, which is mostly visible light, it heats up. Like the sun, Earth emits energy––but because it is cooler than the sun, Earth emits lower-energy infrared wavelengths. Greenhouse gases in the atmosphere (methane, carbon dioxide, etc.) let visible light pass through, but absorb infrared light––causing the atmosphere to heat up. The warmer atmosphere emits more infrared light, which tends to be re-absorbed––perhaps many times––before the energy eventually returns to space. The extra time this energy hangs around has helped keep Earth warm enough to support life as we know it. (In contrast, the moon has no atmosphere, and it is colder than Earth, on average.)
Since the industrial age began around the year 1750, atmospheric carbon dioxide has increased by 40% and methane has increased by 150%. Such increases cause extra infrared light absorption, further heating Earth above its typical temperature range (even as energy from the sun stays basically the same). In other words, energy that gets to Earth has an even harder time leaving it, causing Earth’s average temperature to increase–– producing global climate change.
[In molecular detail, greenhouse gases absorb infrared light because their molecules can vibrate to produce asymmetric distributions of electric charge, which match the energy levels of various infrared wavelengths. In contrast, non-greenhouse gases (such as oxygen and nitrogen–– that is, O2 and N2) don’t absorb infrared light, because they have symmetric charge distributions even when vibrating.]
34-word Summary:
Earth transforms sunlight’s visible light energy into infrared light energy, which leaves Earth slowly because it is absorbed by greenhouse gases. When people produce greenhouse gases, energy leaves Earth even more slowly––raising Earth’s temperature.
88-word Summary:
(a) Earth absorbs most of the sunlight it receives; (b) Earth then emits the absorbed light’s energy as infrared light; (c) greenhouse gases absorb a lot of the infrared light before it can leave our atmosphere; (d) being absorbed slows the rate at which energy escapes to space; and (e) the slower passage of energy heats up the atmosphere, water, and ground. By increasing the amount of greenhouse gases in the atmosphere, humans are increasing the atmosphere’s absorption of infrared light, thereby warming Earth and disrupting global climate patterns.
Reference:
Michael Andrew Ranney, et al., Improving Americans’ Modest Global Warming Knowledge in Light of RTMD (Reinforced Theistic Manifest Destiny Theory). From “The Future of Learning: Proceedings of the Tenth International Conference of the Learning Sciences, Volume 2” (pp. 2-481 to 2-482), edited by J. van Aalst, K. Thompson, M. M. Jacobson, & P. Reimann. International Society of the Learning Sciences, Inc.
http://hamschank.com/convinceme/downloads/papers/RanneyEtAl-ICLS2012.pdf
Related paper:
Michael Andrew Ranney, et al. Changing Global Warming Beliefs with Scientific Information: Knowledge, Attitudes, and RTMD (Reinforced Theistic Manifest Destiny Theory). From “Proceedings of the 34th Annual Meeting of the Cognitive Science Society” (pp. 2228-2233), edited by N. Miyake, D. Peebles, & R. P. Cooper. Austin TX: Cognitive Science Society.
http://hamschank.com/convinceme/downloads/papers/RanneyEtAl-CogSci2012.pdf
Here is an excerpt from the paper that gives and indication of a result from the 2012 study:
“…Across all pre-tests, not a student mentioned different light/radiation types or atmospheric retention time, despite an explicit prompt to explain any differences between the energy moving toward and away from Earth. After reading the 400-word description, though, 61% of Berkeley students (and 55% of Brownsville students) across both conditions correctly answered that Earth emitted infrared light. We also found dramatic increases in true (scored) GW [global warming] knowledge….”Here is video explanation of global warming (a bit over a minute long) from http://www.howglobalwarmingworks.org:

CC1.1. Investigation:
Light in the Atmosphere
A game that models greenhouse effect.

CC1.2. Investigation: Podcasts and Videos
Tune in to the Yale Climate Connections podcasts produced by the Yale Center for Environmental Communication (YCEC). The series aims to help listeners understand how climate change is impacting our lives and what diverse people and organizations are doing to reduce the associated risks.
Also check out Earth: The Operators’ Manual. Host Richard Alley, takes viewers on a High-Definition trip around the globe, from New Zealand to New Orleans, telling the story of Earth’s climate history and our relationship with fossil fuels.
And don’t miss this youtube video: Carl Sagan testifying before Congress in 1985 on climate change and the greenhouse effect. [https://www.youtube.com/watch?v=Wp-WiNXH6hI]

